Economic Evaluation of Pharmaceuticals: Pharmacoeconomics and Health Outcomes
Commentary - (2024) Volume 13, Issue 2
Description
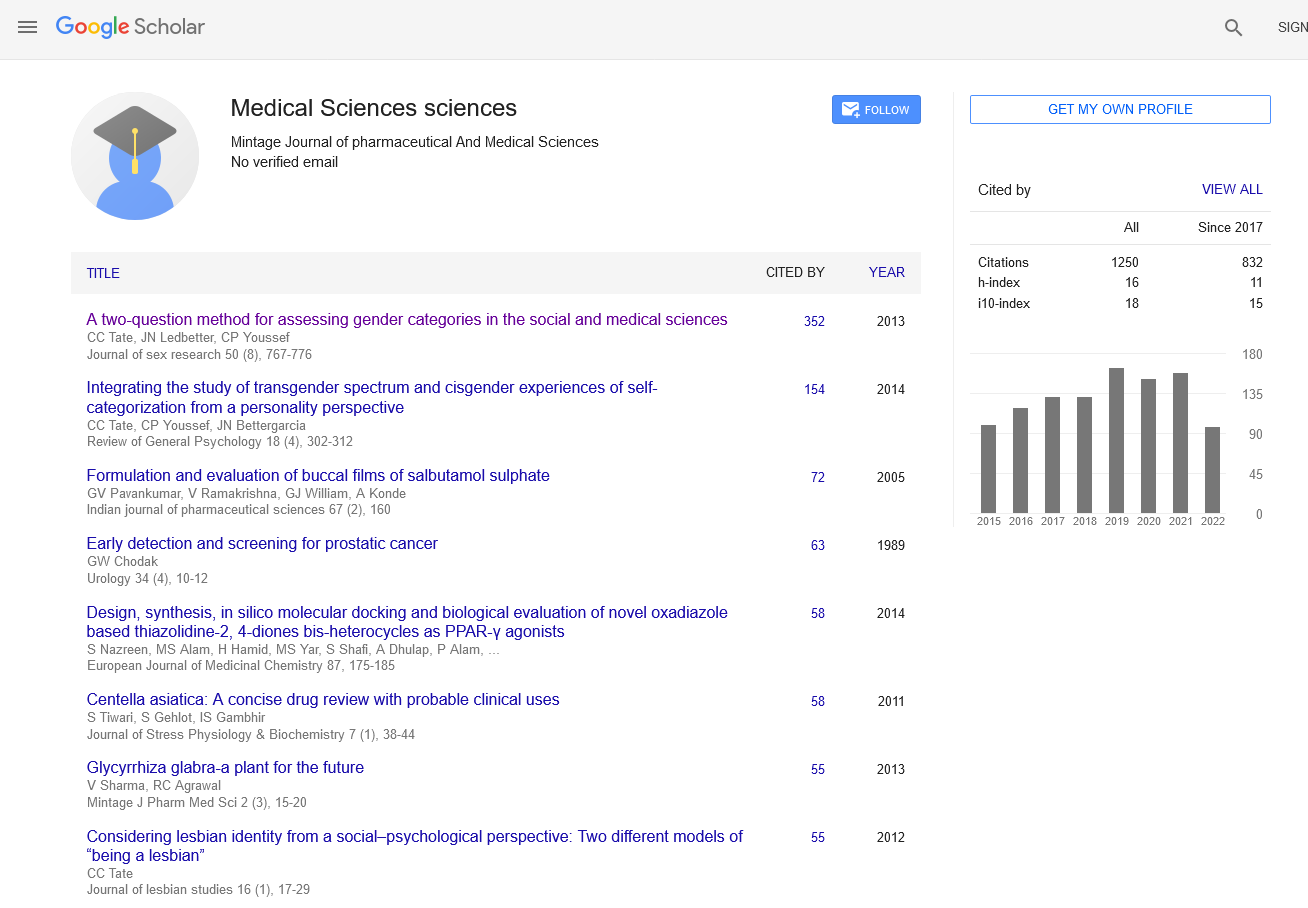
Economic evaluation in pharmaceuticals, specifically through pharmacoeconomics, plays a crucial role in determining the value and costeffectiveness of healthcare interventions. This field integrates economic principles with clinical outcomes to inform decision-making processes regarding drug therapies, resource allocation, and healthcare policy. Pharmacoeconomics examines the costs and outcomes associated with pharmaceutical treatments to assess their economic efficiency and societal impact. It encompasses various methodologies to analyze factors such as cost-effectiveness, cost-utility, and cost-benefit ratios. These evaluations help healthcare systems prioritize interventions that provide the greatest health benefits relative to their costs, thereby optimizing the allocation of limited resources. One of the primary metrics used in pharmacoeconomic analyses is cost-effectiveness analysis (CEA). CEA compares the costs of a pharmaceutical intervention with its clinical outcomes, often measured in terms of health gains or improvements in quality-adjusted life years (QALYs). By quantifying the cost per unit of health outcome achieved, decision-makers can assess whether a treatment represents value for money compared to alternative therapies or standard care. Another approach, cost-utility analysis (CUA), extends beyond clinical outcomes to incorporate patients’ quality of life. It evaluates interventions based on their ability to improve health-related quality of life, often expressed as QALYs gained. This method provides a broader perspective on the overall impact of pharmaceutical treatments on patients’ well-being and societal welfare. Furthermore, pharmacoeconomic evaluations consider both direct and indirect costs associated with pharmaceutical interventions. Direct costs include expenses related to drug acquisition, administration, and monitoring, as well as costs avoided by preventing disease progression or complications. Indirect costs, such as productivity losses due to illness or disability, are also factored in to provide a comprehensive economic assessment. The findings of pharmacoeconomic studies are essential for healthcare decision-makers, including policymakers, insurers, and healthcare providers. They inform reimbursement decisions, formulary management, and pricing strategies, influencing the accessibility and affordability of pharmaceutical treatments within healthcare systems. By identifying cost-effective interventions, pharmacoeconomics helps optimize healthcare spending while maximizing health outcomes for populations. Moreover, pharmacoeconomic research contributes to evidence-based medicine by integrating economic considerations into clinical practice guidelines and treatment protocols. It fosters a systematic approach to evaluating the value of pharmaceutical innovations and therapies, ensuring that healthcare resources are allocated efficiently to achieve optimal health benefits. In conclusion, pharmacoeconomics plays a pivotal role in the evaluation and appraisal of pharmaceuticals within healthcare systems. By quantifying the economic impact and health outcomes of drug therapies, it facilitates informed decision-making, supports healthcare resource allocation, and promotes cost-effective healthcare delivery. As healthcare continues to evolve, pharmacoeconomic principles will remain integral to shaping policies and practices that enhance patient care and healthcare sustainability. First, comprehensive medication reconciliation is essential to identify potential interactions before initiating FXI inhibitor therapy. Second, ongoing monitoring of coagulation parameters and clinical signs of bleeding or thrombosis can help detect and manage interactions early. Third, dose adjustments and alternative therapies should be considered based on the interaction potential and patient-specific factors. In conclusion, while FXI inhibitors offer a promising anticoagulant option with potentially lower bleeding risks, the clinical relevance of drug-drug interactions cannot be overlooked. Careful consideration of concomitant medications, patient-specific factors, and vigilant monitoring are essential to optimize the safe and effective use of FXI inhibitors in clinical practice. By understanding and managing these interactions, healthcare providers can better navigate the complexities of anticoagulation therapy, enhancing patient outcomes.
Acknowledgement
The authors are very thankful and honoured to publish this article in the respective Journal and are also very great full to the reviewers for their positive response to this article publication.
Conflict Of Interest
We have no conflict of interests to disclose and the manuscript has been read and approved by all named authors.
Author Info
Tadahito Yasuda*Received: 29-May-2024, Manuscript No. mjpms-24-141861; , Pre QC No. mjpms-24-141861 (PQ); Editor assigned: 31-May-2024, Pre QC No. mjpms-24-141861 (PQ); Reviewed: 14-Jun-2024, QC No. mjpms-24-141861; Revised: 19-Jun-2024, Manuscript No. mjpms-24-141861 (R); Published: 26-Jun-2024, DOI: 10.4303/2320-3315/236018
Copyright: © 2024 This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

ISSN: 2320-3315
ICV :81.58