The Role of Intravenous Lipid Emulsions in Managing Anticonvulsant Toxicity
Commentary - (2024) Volume 13, Issue 1
Description
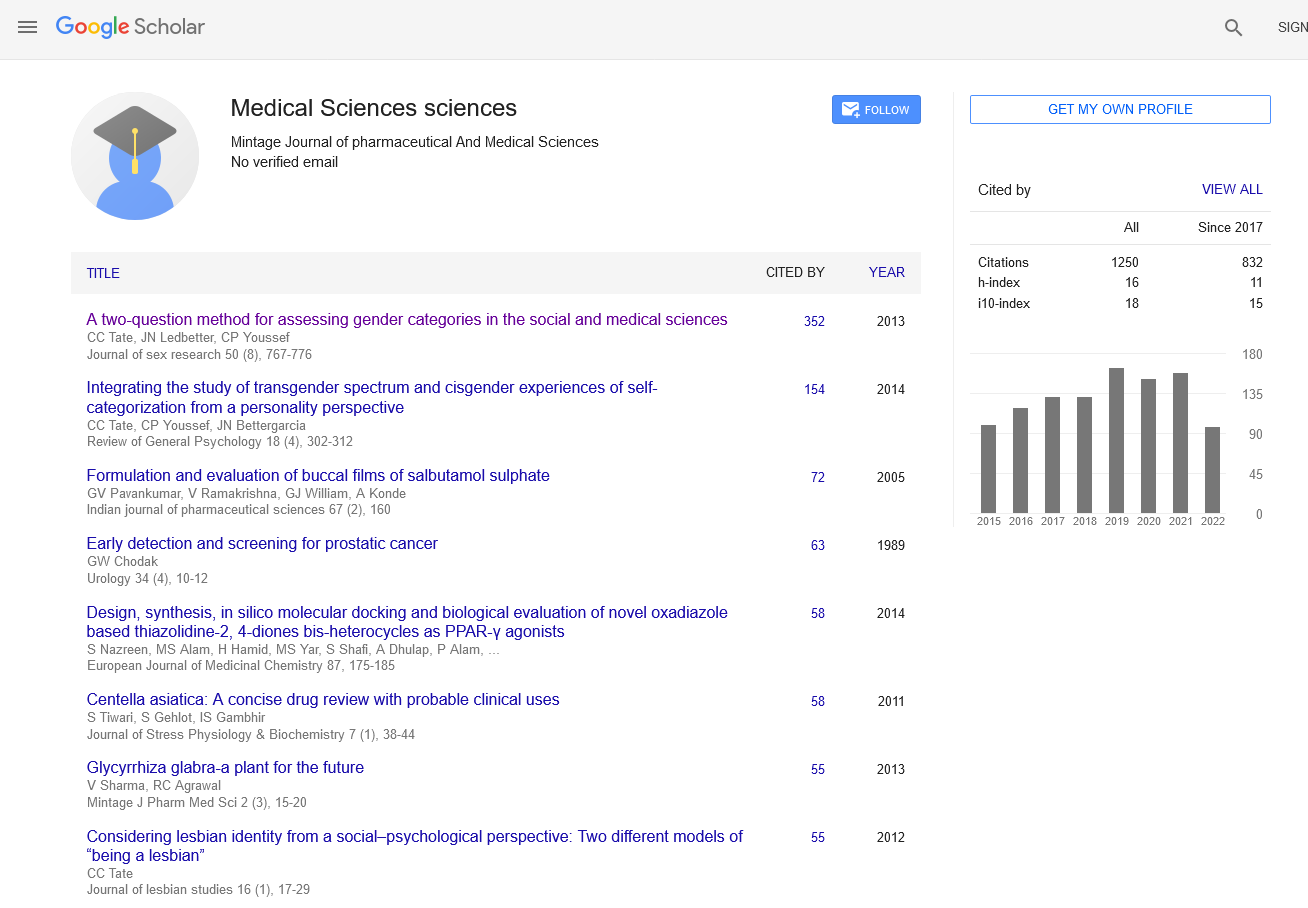
Factor XI (FXI) inhibitors are emerging anticoagulants that show promise in reducing the risk of thromboembolic events with potentially lower bleeding risks compared to traditional anticoagulants like warfarin and direct oral anticoagulants (DOACs). However, as with any medication, the potential for drug-drug interactions (DDIs) remains a critical consideration in clinical practice. Understanding these interactions is essential for optimizing therapy and ensuring patient safety. FXI inhibitors, such as abelacimab and osocimab, work by targeting Factor XIa, a key enzyme in the intrinsic pathway of the coagulation cascade. While this mechanism provides effective anticoagulation, it also opens the door to interactions with other drugs that patients may be concurrently using. These interactions can alter the pharmacokinetics (absorption, distribution, metabolism, and excretion) or pharmacodynamics (the effects of the drug on the body) of FXI inhibitors, leading to either reduced efficacy or increased risk of adverse effects. One major area of concern is the interaction between FXI inhibitors and drugs that affect the cytochrome P450 (CYP) enzyme system, particularly CYP3A4. Many drugs, including certain antibiotics (like clarithromycin), antifungals (like ketoconazole), and HIV protease inhibitors (like ritonavir), are potent inhibitors or inducers of CYP3A4. If an FXI inhibitor is metabolized by this pathway, co-administration with these agents could lead to significant changes in the drug’s plasma levels. For instance, CYP3A4 inhibitors could increase FXI inhibitor levels, raising the risk of bleeding, while CYP3A4 inducers could decrease its levels, reducing its anticoagulant efficacy. Another important consideration is the interaction with other anticoagulants and antiplatelet agents. Patients with cardiovascular conditions often require multiple antithrombotic therapies, increasing the risk of bleeding. The concurrent use of FXI inhibitors with drugs like aspirin, clopidogrel, or other anticoagulants such as warfarin or DOACs can enhance anticoagulant effects, necessitating careful monitoring and possible dose adjustments. Physicians must weigh the benefits of combined therapy against the heightened bleeding risk, especially in patients with a history of gastrointestinal bleeding or those undergoing invasive procedures. Nonsteroidal anti-inflammatory drugs (NSAIDs) also present a potential interaction risk. NSAIDs, commonly used for pain and inflammation, can impair platelet function and cause gastrointestinal irritation, compounding the bleeding risk when used with FXI inhibitors. Monitoring and possibly limiting NSAID use in patients on FXI inhibitors is advisable to mitigate this risk. The interaction between FXI inhibitors and cardiovascular medications, such as statins, is another aspect of clinical relevance. Statins are metabolized by CYP3A4, and their interaction with FXI inhibitors could affect statin levels, potentially leading to either suboptimal lipid control or increased statin-related side effects like myopathy. While current data on FXI inhibitors and statin interactions are limited, clinicians should remain vigilant and consider alternative lipid-lowering therapies if necessary. Furthermore, the polypharmacy common in elderly patients increases the likelihood of DDIs. Older patients often have multiple comorbidities requiring complex medication regimens, heightening the importance of careful medication review and management. Renal and hepatic function decline with age, which can affect drug metabolism and excretion, further complicating the management of potential DDIs. To mitigate the risks associated with DDIs in patients using FXI inhibitors, several strategies can be employed. First, comprehensive medication reconciliation is essential to identify potential interactions before initiating FXI inhibitor therapy. Second, ongoing monitoring of coagulation parameters and clinical signs of bleeding or thrombosis can help detect and manage interactions early. Third, dose adjustments and alternative therapies should be considered based on the interaction potential and patient-specific factors. In conclusion, while FXI inhibitors offer a promising anticoagulant option with potentially lower bleeding risks, the clinical relevance of drugdrug interactions cannot be overlooked. Careful consideration of concomitant medications, patient-specific factors, and vigilant monitoring are essential to optimize the safe and effective use of FXI inhibitors in clinical practice. By understanding and managing these interactions, healthcare providers can better navigate the complexities of anticoagulation therapy, enhancing patient outcomes.
Acknowledgement
The authors are very thankful and honoured to publish this article in the respective Journal and are also very great full to the reviewers for their positive response to this article publication.
Conflict Of Interest
We have no conflict of interests to disclose and the manuscript has been read and approved by all named authors.
Author Info
Victor Newhouse*Received: 28-Feb-2024, Manuscript No. mjpms-24-136523; , Pre QC No. mjpms-24-136523 (PQ); Editor assigned: 01-Mar-2024, Pre QC No. mjpms-24-136523 (PQ); Reviewed: 15-Mar-2024, QC No. mjpms-24-136523; Revised: 20-Mar-2024, Manuscript No. mjpms-24-136523 (R); Published: 27-Mar-2024, DOI: 10.4303/2320-3315/236010
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

ISSN: 2320-3315
ICV :81.58